O příspěvku
Centrum Nauk Przyrodniczych w Publicznym Gimnazjum Nr 6 w Opolu
Abstract
We are going to show how we integrate the content of physics, mathematics, chemistry and biology with the humanistic subjects during our lectures in the Natural Science Center. The Center was established in the Public Junior High School No. 6 in Opole in the school year 2011/2012.
Teachers who are conducting classes have a common theme – the air.
We will present a short video that will describe this element and posters which show selected experiences that we practise during our classes.
The Natural Science Center in Opole
The Natural Science Centre is the first institution of this kind which is acting at school in Poland. Through coming years we will be carrying out project ‚Four Elements‘. This year we focused on the first element - air. Classes are conducted for kindergartens, primary schools and junior high schools. For the new school year also for children with physical disabilities. Pupils from our school will help them during the experiences.
All participants take part in the cycle of four classes of biology, chemistry, physics and mathematics. For these classes there are coming organized groups from all kinds of schools. On Saturdays, there are open classes for pupils and their parents.
During the all classes, the experiments with available devices are performed and its simple versions with use objects and materials of everyday use was prepared, which pupils can repeat at home.
Polish – The film "Four Elements" is an introduction to discussed issues. In the framework of the project implementation ”Four Elements”, which assumes integration between the subjects and joins natural sciences with humanistic ones, teachers and pupils created the film ”Four Elements”.
To reach this target there were used photographs available on the internet. Four elements were presented from the smallest particles – e.g. lumps of earth, droplets of water, sparks of the fire, bubbles of air – to phenomena in the macro dimension of the river, seas, oceans, fields of the earth seen from the flight of a bird, fires absorbing vast areas, hurricanes and whirlwinds constituting a real danger for the man.
Carefully selected music had to illustrate the shown element. The text was drawn up together with Mrs Bożena Jeluków.
The whole film was carried out with using the straight in service the Movie Maker program (by Anna Trzebuniak).
Model plots handed over on classes at individual subjects and descriptions of experiences illustrating discussed issues.
Biology, chemistry, physics
The earth's atmosphere – held thanks to the gravity. It is a combination of gases: nitrogen (78 %), oxygen (21 %), and 1 % of argon, carbon dioxide, helium, neon light, krypton, xenon, methane and hydrogen. If the planet has the same size as an orange, then the thickness of her atmosphere would be equal to the thickness of the paper in which it was wrapped up.
Along with the increase of the height the atmosphere is undergoing the rarefaction: the 80 % of her mass is falling on bottom 10 km (at this height air isn't suitable already for any respiration). There is already only 1 % of her mass above 30 km. By 800 km on 1 cm3 only 106 atoms are falling to the height (gas rules aren't applicable here), above 1500 km 1 atom is falling on 1 cm3 – this density is comparable with the density of interplanetary vacuum
The atmosphere is divided into 5 layers:
Troposphere – here all physical phenomena affecting the weather are reached to the about 11 km. Being a part of the stratosphere heights from 19 to 23 km is particularly rich in ozone. It is called the ozone layer and plays an important role in the safeguard of all living organisms against the detrimental action of the ultraviolet radiation, emitted by the Sun
In this layer the temperature is decreasing monotonously with the increasing in the height about 0,6°C/100 m and on the upper limit of the troposphere achieves value from -55 °C (above polar areas) to -80 °C (above equatorial areas).
Stratosphere – to the about 50 km, in this area a majority of long-range and military planes are moving. There is the ozone layer which is consuming a large portion of the energy of the sun. It contributes to the temperature rise in the height of about 50 km, and it achieves positive values.
Mesosphere – up to the about 80 km – a low temperature rules in it but air is still enough thick that falling meteors can be burnt in it. In mesosphere the aurora appears. The air temperature is decreasing with increasing of the altitude.
Thermosphere (ionosphere) – up to the about 800 km – in this layer space shuttles orbit (350 km), the temperature is rising in relation to absorbing the solar radiation and achieves values higher than 1500 °C).
Exosphere above – 800 km the most outside layer of the earth's atmosphere consists mainly of hydrogen and helium. The temperature is decreasing close to the absolute zero (-273,15 °C). It is going into the interplanetary space.
Possibility of existing on various heights
Simple Respiration – 100 % of oxygen on the sea level, where the human organism is functioning normally.
Heavy breathing – 75 % of oxygen on the height of 2500 m above sea level (e.g. Rysy – Tatry Mountains of 2499 m above sea levels). At this height every organism feels the influence of thin air. Breath becomes faster because there is less oxygen in blood. The brain is swelling slightly, causing headaches and nausea.
Civilization stops here – 50 % of oxygen at 5000 m above sea level. The highest town in the world is Rinconada in Peru (5099 m a.s.l.). There is about 30 000 inhabitants. Above this point there aren’t any human settlements.
No man is able to adapt of one's organism for all-year-round staying in conditions prevailing above 5000 m a.s.l. Lungs expels more carbon dioxide, disturbs the balance in the pH process of blood (pH blood becomes more alkaline). Kidneys secrete more water so that blood can reach the appropriate level of acidity. This leads to the organism’s dehydration.
The greater quantity of erythrocytes causes the increasing of the blood viscosity what is hindering the action of the heart. Therefore, people born on the greater height (e.g. Szerpowie – inhabitants of Himalayas in India and Nepal) they have bigger hearts, which pump blood more effectively. Their lungs and tissues have more capillaries so the transport of oxygen is more facilitating.


Zone of the Death – 30 % of oxygen on the height of 8850 m a.s.l. On the Earth, there are fourteen of such peaks – 10 in the Himalayas and 4 in Karakorum. On the Mount Everest (8848 m a.s.l.) the pressure is taking out about 280 hPa, that is approximately 3,5 times smaller than on the sea level.
Therefore Himalayan mountaineers have pains and the giddiness, palpitation of the heart – even during the rest, the sleeplessness, the poor appetite, irritation, pains in the muscles, nausea, vomiting, swelling on the face, hands and feet, problems with the miction. Lowering the pressure surrounded by the man causes the gasses’ expansion contained in digestive tract, middle ear, imprecisely filled dental losses. As a result of it there appear health problems like: flatulences, intestinal colics, earaches, teeth aches and bay aches.
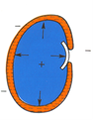
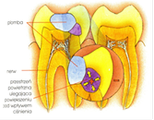
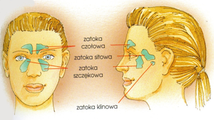
During the violent and big decrease of pressure, gasses expand suddenly in lungs. It can lead to pulmonary oedemas. A pulmonary tissue can be damaged. However the cerebral oedema is responsible for hallucinations – seeing apparitions or hearing sounds, e.g. orchestras.
Physics and biology
Experiment 1
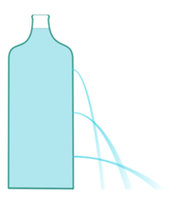
In the plastic bottle we do three holes with the needle heated up. Next we fill the bottle up with water and observe reaches of streams flowing out from individual holes.
The pressure decreases with altitude.
Experiment 2
Empty bottle made of soft plastic is filling with hot water up to the about 2–3 cm from the bottom, then we turn it off. Next we cool down the bottle in the cold water. The pressure inside is decreasing so the external pressure is crumpling the bottle.
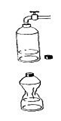
Experiment 2a
Into the empty can we are pouring waters into the about 1 cm and we warm it on the gas cooker (bringing the water to the boil). Next with pliers we should take the can from the burner and turn it away, then we plug it in the bowl with the cold water. The can with a rumble is crushed.
Experiment 2b
We fix a car valve to a cap of 5 litre bottle. We put a little balloon into the bottle and pump it to make it touch bottle’s walls. Then we turn on a cap and with a car pump we compress air in a bottle until a balloon starts to fall.
The pressure higher than the atmospheric one is able to crumble us.


Experiment 3
Under the lampshade of a vacuum pump we put the pigs’ lungs. After pumping air out lungs enlarge greatly.


Experiment 3a
Into the 2 litre bottle made of the hard plastic we cut the bottom and put on it the thin rubber glove which will perform the role of the diaphragm. Next we fit the cork with two holes in which we put two glass tubes. To the ends of the tubes we fix two balloons. Next we cork it tightly, this way that balloons nailed to tubes are found inside the bottle. We demonstrate the rule of operation of lungs: stretched glove – lowered pressure inside the bottle – lungs are increasing in their volume, not stretched glove – lungs are reducing their volume.
Experiment 3a was presented by Italians during Physics on Stage 3 in Netherlands 2003.


Experiment 4
We put a glass filled with 70 °C water under the cover of vacuum pump. After the air is pumped out water boils in the reduced pressure.

Another danger is the phenomenon of boiling body liquids, which may be dangerous in stratospheric flights. On the height of 19,2 km the pressure is so low that the temperature of boiling water equates 37 °C. It is relevant for the temperature of human body. We can deduce then, that on this height human body liquid can be boiled. Such experiments were run on animals in pressure chambers.
Experiment 5
In plastic 0,5 litre bottle we do the small hole (on the height of the about cm from the bottom). We pull the 10 cm rubber tube through the screw cap of the bottle. In the bottle we stop the hole, pouring waters into it, and to the rubber tube we put the cigarette. After light the cigarette, we remove the stopper in the bottle. Water leaking out causes sucking of the cigarette smoke. When water leaks out, we put the cigarette out, and then we twist the bottle off. We take a clean piece of cotton and we pour out the water which stayed at the bottom on it. The chemical substances contained in the cigarette smoke will settle on cotton.
http://patrz.pl/filmy/jak-oduczyc-sie-palenia


Chemistry
Detection of air
Experiment 1 (Detection of air)
Into the plastic bottle we put the rubber balloon and we try to fill it with the exhaled air. Next into the same bottle we put the tube to drinks and again we blow the balloon up. In both cases we are observing the degree of filling the balloon.



Experiment 2
We are filling the big dish with water. From a sheet of paper we shape the ball and put it at the bottom of the plastic small mug. We invert the cup upside down and submerge in a bowl of water. After taking the mug out we are checking how the paper ball looks like.
Weighing the air
Experiment 3
We are putting two glass bottles closed tightly on small scale pans of the food scale with vacuum corks. Using weights we lead the weight to the equilibrium position.Using a vacuum pump from one bottle we are pumping air off. Then again we lead the weight to the equilibrium position.

Appointing the density of air
Experiment 4
We are filling the bottle from previous experience up with water and we close it tightly with vacuum cork. Next we are pouring water to the cylinder and read the volume of liquid out equivalent of capacity of the bottle. Then we calculate the density of air dividing the air mass appointed in previous experience by the volume the bottle.
Mathematics – How many walls does the air have?
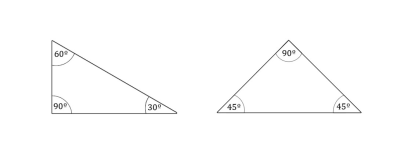
Class topic like “How many walls does the air have” forces all attendees to resist. Have you ever heard about such thing? The air having walls? The answer could be found in ancient times! Many ancient cultures gave already some theories according to which the whole world is build up from basic elements. Plato (427-347 B.C.) presented his Geometric Atomism Theory in which he marked two geometrical forms to be the basics matter structure. To be precise two types of right-angled triangles: equilateral right-angled triangle and different-arm right-angled triangle with 30, 60 and 90 degree angles. He explained the variety of matter due to infinite number of those elements possible combinations.
Folding appropriate amounts of right-angled triangles of one kind, participants in classes are creating arrangements called by the Plato “mathematical elements”.
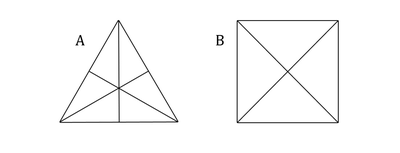
First from elements, equilateral triangle is an object of pupils’ further action. Students using play dough and plasticine should build equilateral triangles.
| Resources |
Task |
| 3 sticks |
One equilateral triangle |
| 4 sticks |
Two equilateral triangles |
| 6 sticks |
Four equilateral triangles |
To obtain a tetrahedron – the first of Plato solids use six bars – create four equilateral triangles. Plato thought that the world consists of four basic elements like: water, fire, earth and air which are built from corresponding regular polyhedrons.
Fire – tetrahedron
Earth – hexahedron
Air – octahedron
Cosmos – dodecahedron
Water – icosahedron

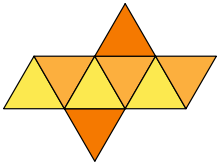
Assuming the theoretical part the answer for given question seems to be quite clear.
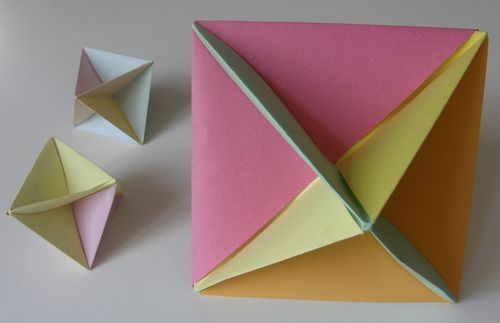
Creating an octahedron model made from previously built mesh is definitely an educative task; additionally your spatial intelligence will be strongly used and even improved.
The air models can be done due to origami technique (www.matematyka.wroc.pl)
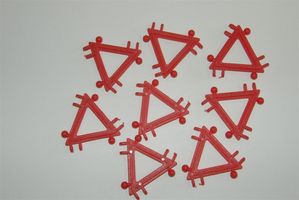
The easiest octahedron model version may be made from REGO blocks.

References:
[1] D. Tokar, B. Pędzisz, B. Tokar; Doświadczenia z fizyki dla szkoły podstawowej, z wykorzystaniem przedmiotów codziennego użytku, Wydawnictwa Szkolne i Pedagogiczne, Warszawa 1990
[2] National Geographic, maj 2003. s. 98
[3] Współczesny świat w nauce; Świat Książki, Warszawa 2003, s.290
[4] Dougal Dixon; Encyklopedia „Wiedzy i Życia" , „Atmosfery i oceany”, Wiedza i Życie 1991, s.22.
[5] Robert J. Brown; 200 doświadczeń dla dzieci, Wydawnictwo Prószyński i Spółka, Warszawa 1999
[6] Hermann Krekeler, Marlies Rieper-Bastian Fascynujące eksperymenty łatwe, odkrywcze, zaskakujące, red. Katarzyna Andrzejczuk, Warszawa 2002
[7] Janice Van Cleave 101 ciekawych doświadczeń, Wydawnictwo Szkolne i Pedagogiczne, Warszawa 1993
[8] Bertrand Russell, Dzieje filozofii Zachodu; Fundacja Aletheia 2000
[9] Anne Rooney, Fascynująca matematyka; Bellona 2011
[10] Małgorzata Mikołajczyk, Ośmiościan dla leniwych; www.matematyka.wroc.pl http://meteorologiaonline.republika.pl/atmosfera.htm
[11] Mountain A; Nurkowanie; Galaktyka, Łódź 2003