About paper
Czech originalIce – The Physicist’s friend
Abstract
Water is one of the most fantastic materials in the universe in its solid state as well as in its liquid state. This contribution is concentrated on studying the basic properties of ice in both demonstrative experiments and in experimental homework.
The water anomaly
Water is a material that we see a lot and hence it mostly does not cross our mind that water is very special. Let me introduce here a few properties, in which it markedly differs from chemically comparable materials (see reference [1], where you can find 37 more differences and also their explanations): water has a high melting point, high boiling point, high surface tension, high viscosity (that moreover decreases with increasing pressure), during melting it increases its specific density, between 0 °C and 4 °C its density with increasing temperature increases, and it can be supercooled till -70 °C (in small drops).
Volume changes during melting and solidification
Unlike most materials water increases its volume during solidification. One of the easiest demonstrations of this fact it is uses a refrigerated PET bottle. Fill any PET bottle with water, close it with a cap (screw top) and put it in a freezer for 24 hours. The frozen water strongly deforms the bottle (in experiments where the PET bottle is pressurized with air a similar deformation is seen with pressure above 300 kPa). If you let water freeze in a tetrapack box with a screw cap, the ice will split it. You should never do these experiments with a glass bottle! It is not pleasant work to clean up the freezer from slivers of glass.
As homework you can ask the pupils to measure the change of water’s volume during solidification. Good for use is a 20 ml syringe, where you put 15 ml of water in and then put the syringe in a freezer (no need to use a needle). Syringes are made from durable plastic, hence the ice does not dilate it, but moves the piston of the syringe to the mark 16 ml. This corresponds to volume increasing by 1/15 = 7 % (the more accurate value is 8.3 %).
Students mostly know this property of water, but they do not expect other materials to have the opposite behaviour. To demonstrate this we can use wax. We dissolve wax in a preserve jar that is immersed in boiling water and then pour wax into a test tube. During solidification the surface of the wax declines markedly – its volume has decreased.
Similarly we can demonstrate that few solid materials float on their own liquid form – into one glass we pour water and throw in ice cubes – the ice floats on the surface of the water (this is a well-known effect). In the next glass we pour melted white wax and throw inside a piece of tough solid coloured wax – it goes to the bottom.
Ice regelation
In older papers(e.g. [2]) ice regelation was usually described as “alloying”. It can be described as refreezing of two separated ice pieces. We can make one nice experiment (described in [2]): bring two big pieces of ice (or you can break a bigger piece in two) and throw them into a vessel with hot water (but not boiling water, as we have to put our hands in it). Then, take both pieces of ice and press them together for a minute – they join together very firmly.
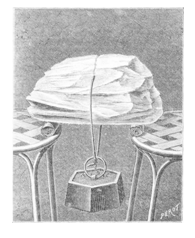
At first sight the effect is surprising, but the explanation is simple – unmelted ice has still a temperature below the freezing point. After pressing two pieces of ice together, the layer of water that stayed between these pieces freezes, because it is not encompassed by hot water, but by cold ice. Hence the pieces of ice refreeze to each other.
When the teacher of physics hears the word “regelation”, they probably recall a classical experiment, where a thin wire with a heavy weight attached to its ends slowly passes through ice-block and the wire’s track is refilled. This experiment you can see in figure [2], which was taken from the French magazine “La Nature”.
A modern version of this experiment can be done using 2-liter PET bottles filled up with water as weights attached to ends of steel wire (the wire you can buy in modeller’s shops).
The explanation of this experiment was interpreted thus: with increasing pressure the melting point of ice decreases, therefore ice melts underneath the wire and refreezes once the pressure has passed (it comes back to its initial value). A similar effect is observed also in skating (that is why skates have to be sharp). However, in the lobbies of the last few trade fairs there were discussions that maybe everything is a little bit different. One of the ideas, that were mentioned there, was the influence of the heat conductivity of steel wire. I tried to look at whether this effect was important. As I use a steel wire with diameter 0,2mm for this experiment, I went to a fishing shop and I bought some Dynastrong string with the same diameter (it is knitted fishing rope from the fibre Dynema, that is made from GSP = Gel Spun Polyethylene = fibre that is spinned from the gel form of polyethylene).
On one ice-block I put both the wire and string with the same weights – they exert the same pressure. The steel penetrated through ice slowly but surely, whereas the fishing line penetrated only 4 cm under surface, and there stopped and froze. In comparison to steel polyethylene is a heat insulator, hence I thought that the problem was solved. Then I decided to try this experiment under low outside temperature. In April I have at my disposal only a fridge with temperature around +4°C. The wire does not heat up from the surrounding air at this temperature; however the wire still passed through ice-block.
After a month I got a few issues of the journal The Physics Teacher by a fortunate circumstance (thanks to Doc. Trnovi from PedF MU Brno, who arranged their borrowing), where I found an article [3] that took an interest in the same problem.
Here it is written that to decrease the melting point of water by 1°C a 14 MPa pressure is needed. However, the calculated pressure that originated under the wire in our experiment is only 2 MPa which could lead to a decrease in melting point only by 0.1°C. In article [3] “surface melting” explains this effect. Its existence was predicted by Michael Faraday in 1842. According to this hypothesis there exists a thin layer of liquid on the surface of a solid. The liquid layer is also below the melting temperature (this decreases its free surface energy). In the case of ice, the layer is 40 nm thin at 0°C and 0.5nm thin at –35 °C. This layer is the reason why the wire can pass through the ice and it also explains the easy skating on ice at temperatures that are not much below the freezing point. According to the author of this article [3] it is practically impossible to skate on ice at temperature –35 °C – the surface layer of liquid water is too thin - the skates do not glide. He tried it personally in arctic winter weather in Canada.
The experiments by R.R. Gilpin confirmed that this hypothesis is an acceptable explanation of this experiment. In 1980 R.R. Gilpin repeated the experiment by using very small forces acting on wire (hence the decrease of melting point is negligible), and at a temperature that was held under 0 °C. Under these conditions the wire passed through ice.
The freezing of a bottle with water
In winter the year before last I put a balloon filled with water behind the window and I thought that I would have an ice ball in the morning. To my greatest surprise despite the frost only a thin layer of water at the edge was frozen and there was liquid on the inside – an “ice egg” was created. You can make this “ice egg” on your own if you put a balloon filled up with water into a freezer for 5-6 hours.
How is it possible that water inside the balloon does not freeze? We have to realize the processes that proceed when water freezes. Water first cools down to the melting point (or slightly below) and after that water freezes. Both when cooling down and when freezing, water has to transfer a big quantity of heat to its surroundings. At first, water transfers heat to the icy air of freezer, hence it cools down relatively fast to 0°C (the balloon is cooled down steadily from all directions, spontaneous circulation of the water of different temperatures occurs by convection – this ensures the same temperature in the whole balloon).
A thin layer of ice is created at the edge of the balloon. Now, the water inside can transfer the heat only to the ice layer, that has a temperature comparable to water – the heat exchange is nearly stopped. To freeze another layer of water a big amount of heat has to be transferred through the ice layer that is not a good conductor. Moreover, the ice has to cool down below its melting point to be able to take the heat from water.
Hence the formation of a thin ice layer slows down the freezing process dramatically. Water in the ice egg goes into a super-cooled state – despite having a temperature below the melting point it does not freeze. Less amount of heat is needed for transformation to this state.

Despite “common freezing” some super-cooled water freezes inside of the ice egg. When you break this ice egg, you can reveal strange flat fruticose formations that are mutually crossed and fibrillated. One of these formations (the so-called Tyndall) we can see in the figure [4].
If we break the ice egg after being a few days in freezer, we can see 1cm of transparent ice layer at the edge (created by “common freezing”) and a white mushroom-shaped structure inside, that contains not-frozen water. This mushroom-shaped structure was created by overgrowing the flat fruticose formation. For creation and observation of this structure it is good to use a dish made from foamed polystyrene (fruits, desserts or meats are sold on it).
Fill up this dish with water, at least 4 cm, and then put it in the freezer. Due to the insolating properties of plastic water freezes from the top; hence it is the same case as the balloon. At first a transparent ice layer is created and then inside of the supercooled water the fruticose (dendritical) formation are formed (after 4 hours in the freezer). If you are lucky, you can obtain fruticose of an area of 10 or more square centimetres.
Polarization properties of ice crystals
When we put an ice slab (e.g. that is made from frozen surface in water from dish) into two “crossed” polarized filters we find out that the crystals of ice rotate the plane of polarization of light. We can see a nice picture of many small ice crystals– some of them are dark, others are light. The colourful slab can be created by very thin ice crystals. If it is possible to put on the surface the fruitcose formation that originated in super-cooled water, it will all have the same colour – it is a monocrystal.
Comment
The description of the experiment and the value of the latent heat of fusion of ice can be found in [5].
An article “Investigation of heat anomaly of water” that is about the anomaly of water density will be published in the near future in the journal Mathematics-Physics-Informatics.
References:
[1] http://www.lsbu.ac.uk/water [cit 2005-08-21]
[2] Fyzika bez přístrojův. Vesmír, říjen 1881
[3] White J.D.: The Role of Surface Melting in Ice Skating. The Physics Teacher, Vol.30, Nov.1992, s. 495-497
[4] Walker J,: Exotic Patterns Appear in Water When It Is Freezing or Melting. The Scientific American, July 1986
[5] http://fyzweb.cuni.cz/piskac/pokusy/www/energie/tanidrcl.htm [cit 2005-08-22]