About paper
Czech originalInteresting Electrolysis
In this contribution we shall talk about two different ideas in the field of electrolysis. The first one is the simple measuring of the charge of an electron. It is a motivational experiment, which the pupils can do on their own at home. The second idea is from the field of cold nuclear fusion, which (as it seems) is making a comeback and is considered by many to be the hope of future power industry.
Simple electron charge measurement
To measure the charge of an electron qe, we’ll use a plastic syringe (fig. 1a) filled with diluted sulphuric acid. In the average household this can be obtained from a car battery using e.g. a small plastic tube, or a syringe. Only 3-4 drops of this acid are needed, as they will help us create approximately 3 cm3 of solution.
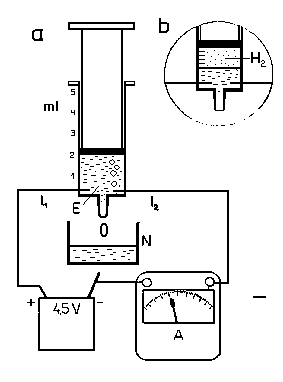
Fig. 1
First we fill the syringe with e.g. 2 cm3 of this solution so that no air is present. We fix the syringe in a vertical position, with the pump pointing upwards. Near the bottom of the syringe two needles will need to be stabbed inside the syringe, these will serve as our electrodes. Then we connect the syringe in an electrical circuit and place a glass container underneath it. Droplets of the solution will drip inside it because of the gas obtained during electrolysis pushing on the solution from above (hydrogen gas, to be precise, it accumulates on the cathode).
A 4.5V battery will serve as our energy source. It takes approximately 5 minutes to create 1 millimetre (1 cm3) of hydrogen gas. It is this gas that pushes part of the solution out of the syringe. The SO4 group created on the anode binds with iron, creating a small FeSO2 layer on the anode; therefore it appears that nothing is happening on the anode. After the layer of hydrogen gas above our solution we interrupt the process (fig. 1b). In order to be more accurate in determining this amount, we use a multiple of 1 millimetre as our starting amount.
The charge qe can be determined from the N amount of separated hydrogen molecules and from the electrical charge Q = It which passed through the circuit over the course of the electrolytic process. We therefore have to measure the current and the duration of electrolysis, I and t, respectively. Each separated molecule corresponds with two electrons passing through the circuit (one electron per separated hydrogen ion). The amount of electrons that passed through the circuit is therefore 2N. From this we can conclude that the complete charge equals Q = 2Nqe. When we compare both sides of the equation for charge, we get 2Nqe = It, which can be derived as \[ q_e = \frac{It}{2N} \] The number of separated molecules can be determined using basic findings of gases. If we don’t wish to determine the charge of an electron too precisely, we can take Loschmit’s number (2.7×1019) as our N amount of hydrogen molecules. We can get this number as well by dividing the Avogadro constant by the volume of 1 mole (under normal circumstances). (A more precise measurement of N requires measuring the temperature and atmospheric pressure).
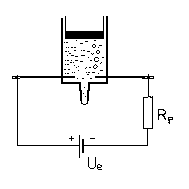
Fig. 2
Measuring without an ammeter
It should be noted that the charge of an eletron can be approximated even without an ammeter. In such a case the ammeter should be replaced with a resistor of known resistance, e.g. 1 kΩ, which is significantly higher than the resistance of our electrolyt (fig. 2). The current can be estimated by using Ohm’s law while ignoring the electrolyte’s resistance. The duration of electrolysis will be longer, however, depending on the voltage of the energy source.