About paper
Czech originalTechnical sprays in physics education
Abstract
Sprays can liven up basic and secondary school physics lessons. Every day we encounter a simple container capable of atomizing its liquid content in the air with various effects: to spray paint, apply a fragrance, disinfect minor wounds, disable attackers etc. Some technical sprays used in electronics such as freeze spray and gas dusters are easily available, affordable and very useful for physics education. Sprays with magnetic particles used by crime scene investigators can be obtained, too.
Airbrush and spray
Artists know the classic airbrush and its principle still can also be found in older physics textbooks.
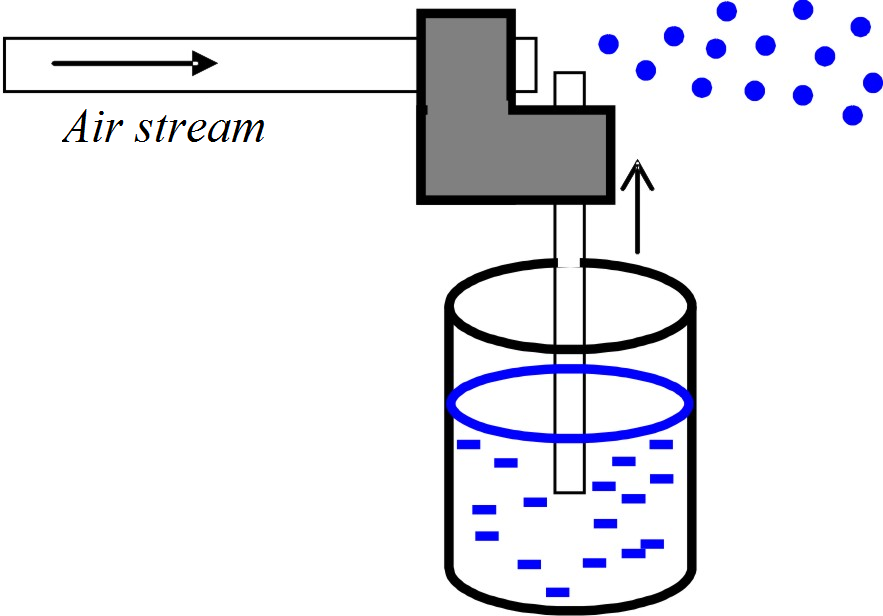
Fig. 1 Classic airbrush
The air flowing past the upper opening of the vertical tube causes a reduction of pressure there and the atmospheric pressure pushes the liquid from the container up the tube. Small droplets of the liquid are torn away by the air stream on the top of the tube and thrown forward. Construction of a simple airbrush with two straws of different diameters can be an interesting assignment for basic school students.
Sprays work without an air stream. The liquid is closed in a container together with the propellant gas. The gas pushes the liquid through a relatively wide tube to a narrow nozzle. The speed of the liquid is increased and its pressure is reduced in the nozzle and it gets atomized. The main physical property of the propellant gas is that its boiling point must be lower than the operating temperature of the spray. There aren’t many suitable gasses.
Propellant gasses
Waterless sprays – paints, lacquers, deodorants, antiperspirants – use a mixture of propane and butane.
Boiling point of propane CH3-CH2-CH3 is −42.1 °C at atmospheric pressure and 0.5 °C for butane CH3-CH2-CH2-CH3. Nevertheless, this mixture is flammable.
Dimethyl-ether CH3-O-CH3 with boiling point −23.6 °C is used in sprays that contain water or ethyl alcohol as a solvent. This group includes many cosmetic sprays, air-fresheners and water-based paints for artists. Dimethyl-ether itself is flammable but not in mixture with water.
Tetrafluorethane CHF2-CHF2 is absolutely non-flammable and has a boiling point of −26 °C. There are more fluoro-hydrocarbons and some of them are used in freeze sprays and gas dusters. They can be mixed together to reach required pressure in the spray. Examples of hydrocarbons with fluorine in the molecule are listed:
HF3C trifluormethane boiling point −81.2 °C;
HF2C difluormethane boiling point −51.6 °C;
CF3-CH3 trifluorethane boiling point −47.75 °C;
CHF2-CH3 difluorethane boiling point −24.7 °C.
Carbon dioxide, nitrogen or air also serve as propellant gasses in some cosmetics sprays. The spray contains a piston or a balloon with the gas in these cases. Shaving foam spray may be interesting for gentlemen – it is propelled by isopentane C5H12 with boiling point 27.7 °C.
Construction of a spray
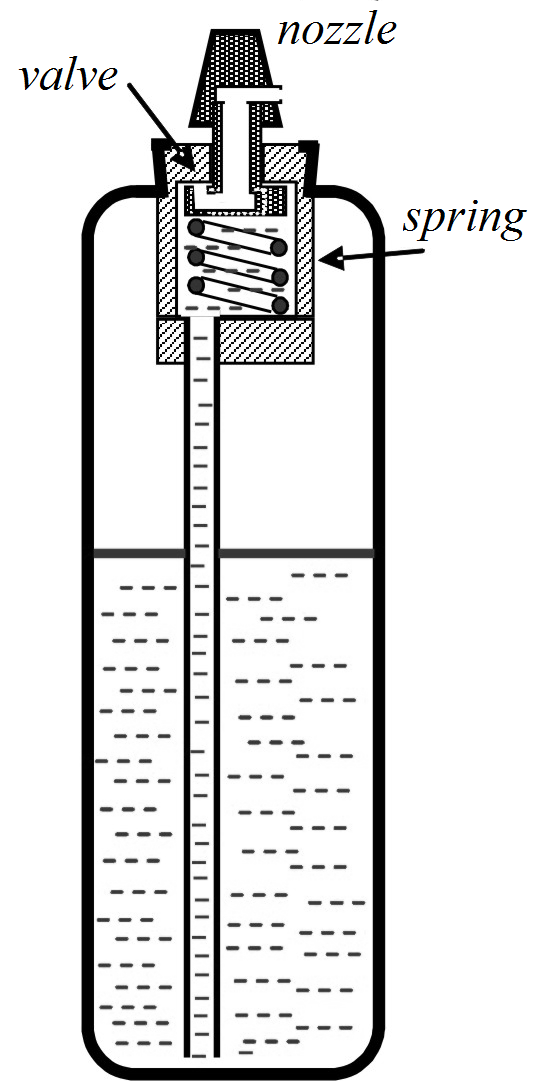
Fig. 2 Cross-section of a spray container
The container contains about two thirds of liquid and one third of propellant gas at pressure up to 0.8 MPa. Pressing of the nozzle opens the valve that is closed by the spring. Figure 2 shows one of the simplest possible constructions.
Sprays with magnetic particles
The liquid in these sprays is a suspension of fine ferromagnetic particles, often dyed or even fluorescent. They can serve to visualise the edges of permanent magnets in spare floppy disk drives, for example:

Fig .3 Rotor of a 5.25″ floppy disk drive
We can see 10 borders between the poles and other 20 borders next to each big pole on the edge of the rotor. The big poles serve as active poles of the synchronous motor (the coils are located in the stator) and the tiny poles give impulses for Hall effect sensors that provide feedback for maintaining desired revolution frequency of the motor. The rotor of a 3.5″ floppy disk drive gives a similar picture (Figure 4).

Fig. 4 Rotor of a 3.5″ floppy disk drive
Sprays with magnetic particles are used by crime scene investigators to reveal the original serial numbers that were ablated by perpetrators. The metal part is placed between magnets and becomes magnetized parallel with its surface. Figure 5 shows how the residual deformation of the original stamping causes local leakage of magnetic flux that attracts the particles.

Fig. 5 Revelation of ablated serial number
Freeze sprays
The common filling of freeze sprays is dimethyl-ether or a mixture of fluoro-hydrocarbons. For example type PRF 101NF (non-flammable) contains 95 to 100 % of tetrafluorethane. The producer claims it can be used to cool down a component to ‑55°C. Next figure shows our own measurement:

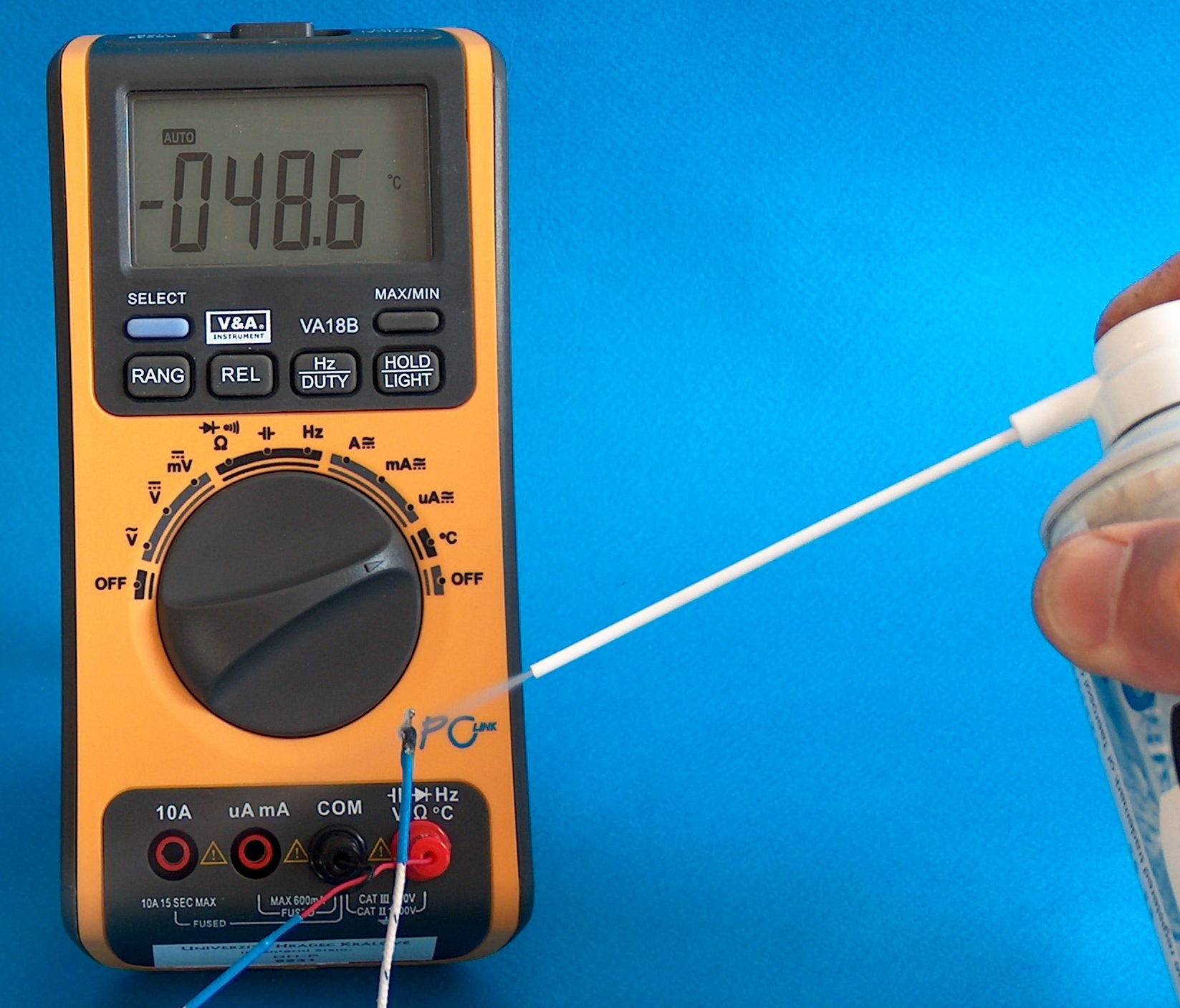
Fig. 6 Cooling with a freeze spray
The temperature of the air in the room is 75.2 °C higher than the temperature on the tip of the thermocouple. One of educational applications is thermal deformation of a plastic ruler in Figure 7.

Fig. 7 Ruler deformation
The upper side of the plastic is contracted and the ruler markedly bends up. Deformation disappears as soon as the whole volume gains the same temperature. There is a layer of ice apparent on the cooled surface that comes from air humidity and melts after a while. The freeze spray is thus useful to show phase transitions.
Thermal expansion is usually demonstrated with positive sign (dilatation by heating). Here we can simply show also contraction by cooling:
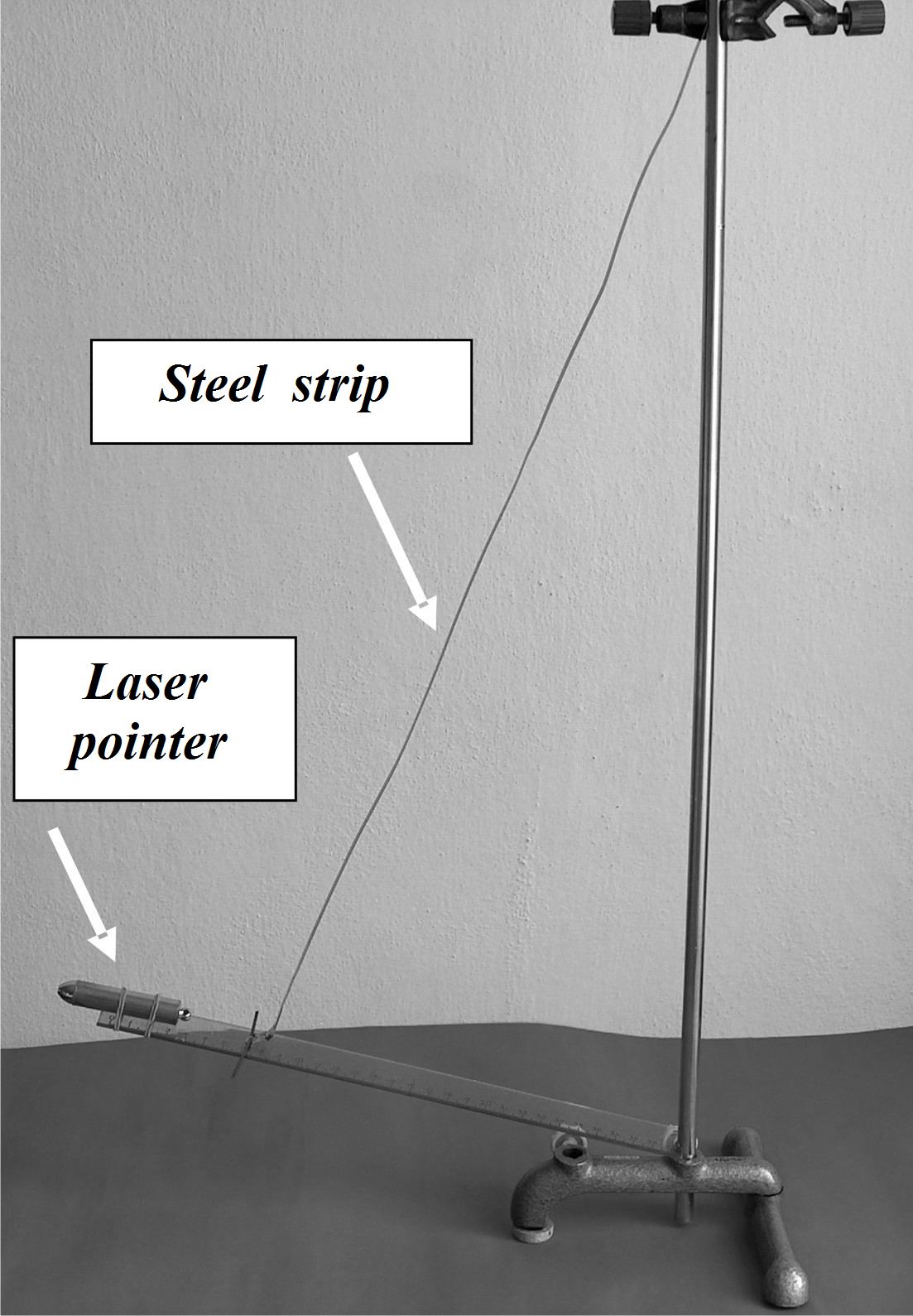
Fig. 8 Set for measuring the contraction of a steel strip by cooling
The laser spot on a wall in the distance of 10 m moved up Δl´ = 5 cm. The steel strip is attached to the pointer arm at radius of 23 cm. Its contraction is \[ \Delta l = \frac{0.23}{10}\,\Delta l' = 0.00115\,\mathrm{m}. \]
We can also calculate the coefficient of thermal expansion if the whole strip is covered with ice and we estimate achieved temperature.
Gas duster
Gas duster is a type of spray used to clean fine mechanics and although it is sometimes called canned air, it usually contains dimethyl ether or tetrafluorethane. Spray DUST OFF 67 contains according to the safety datasheet 10 to 30 % of dimethyl ether and the rest of tetrafluorethane. It provides a physicist with an intensive “air” stream. We can easily demonstrate hanging of a ball – see Figure 9.

Fig. 9 Hanging ball
Where to buy in the Czech Republic
Freeze and cleaning sprays are sold by electronics suppliers GES Electronics (http://www.ges.cz/) and GM Electronic http://www.gme.cz/cz/. Magnetic sprays for non-destructive testing are sold by NDT Trade http://www.ndttrade.cz/164/o-nas-sk.